Abstract
Objective
The marrows and spleens of myelofibrosis (MF) patients are characterized by megakaryocytic (MK) hyperplasia as well as microenvironment (MicroE) abnormalities including increased micro-vessel density, stromal cell (SC) hyperplasia and fibrosis. MF is accompanied by dysregulation of inflammatory cytokines (INF-CyKs) which alter the tissue specific MicroE niches in which MF HSC reside. We hypothesized that MF MKs play a critical role in MF pathobiology and examined their potential to affect MF and normal CD34+ cells as well as marrow and splenic endothelial cells (EC) and SCs.
Methods & Results
MF MKP/MKs elaborate specificINF-CyKs. CD34+ cells were cultured in serum free medium with stem cell factor and thrombopoietin (TPO) for 7 days, and then cultured with TPO alone for an additional 7 days to generate cell population enriched for MK progenitor cells (MKPs) (CD34+CD41+) and MKs (CD34-/CD41+) (MKP/MK: 22-61% in normal and 27.6-54% in MF). MF MKP/MKs as compared to normal MKPs (nMKP/MKs) contained increased transcripts for several INF-CyKs including: IL-8 (31 fold), TGF-β (8 fold) and VEGF (93 fold). The transcripts for the P53 antagonist MDM2 (112 fold) and the activity of the transcription factor NF-κB were also increased. Furthermore, media conditioned by MF MKP/MKs contained increased protein levels of IL-8, TGF-β and VEGF (5.8, 1.4 and 5.2 fold, respectively) as compared to nMKP/MKs. Using immunohistochemistry, we demonstrated that IL-8 protein was present in normal and MF splenic SCs and ECs, but was exclusively present in MF splenic MKs. Plasma IL-8 levels were significantly elevated in MF patient plasma (p=0.0008). These data indicate that MF MKP/MKs elaborate a series of INF-CyKs which promote the development of MF.
IL-8 promotes MF CD34+ cells proliferation. CXCR1 and CXCR2 are two receptors that bind IL-8. Flow cytometric analyses showed that MF CD34+ cells expressed higher levels of CXCR1 and CXCR2 than normal CD34+ cells (3.3 and 3.1 fold, respectively). Addition of IL-8 increased MF CD34+ cell numbers by 2 fold and assayable CFU-GM by 40% (p=0.033), but this effect was eliminated by the addition of reparixin, a CXCR1/CXCR2 antagonist (p=0.0055).
MFMKP/MKs can alter the HSC MicroE. Using IHC and flow cytometry we observed that CXCR1 and CXCR2 were also expressed by splenic SCs and ECs and marrow mesenchymal stem cells. When SCs and ECs were incubated with equal numbers of normal or MF MKP/MKs, higher levels of SCF and VEGF were elaborated by SCs but not ECs. This effect was more pronounced with MF MKP/MKs as compared to nMKP/MKs (p=0.02 for SCF and p=0.003 for VEGF). By contrast, higher levels of IL-8 were elaborated by both ECs and SCs following co-cultivation with MF MKP/MKs (p=0.047 and p=0.03). The addition of reparixin to these co-cultures decreased the levels of VEGF and IL-8 to baseline. Co-culturing MF CD34+ cells with either SCs or ECs significantly increased the numbers of CD34+ cells, an effect which could be blocked by the addition of reparixin. These data indicate that MF MKP/MKs provide inflammatory signals that alter the MF MicroE which supports MF CD34+ cells and that these signals can be disrupted by drugs blocking IL-8-CXCR1/2 interactions.
MFMKP/MKs and CD34+ cells can be targeted with ruxolitinib, the nutlin-RG7112 and a BET inhibitor. Treatment of MF CD34+ cells with low doses of RG7112, ruxolitinib, or JQ1 alone or in combinations decreased MF but not normal hematopoietic colony formation. Treatment of MF MKP/MKs with each of these agents decreased phosphor-NF-kB p65 levels as demonstrated by western blot and decreased the elaboration of IL-8 by 20 to 60%.
Conclusion
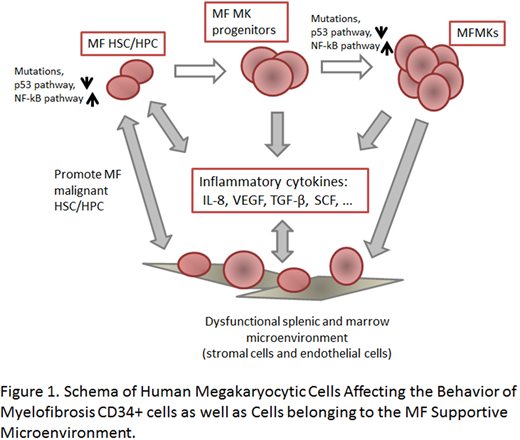
These data indicate that MF MKPs are characterized by increased transcripts for MDM2 as well as NF-κB activity contributing / leading to the MK hyperplasia characteristic of MF and the elaboration of a group of lineage specific cytokines (TGF-β, IL-8, VEGF) that not only affect MF CD34+ cells but also cells belonging to the MicroE (ECs and SCs) (Figure 1). The MF promoting activities of MF MKP/MKs can be effectively targeted with ruxolitinib, RG7112, and BET inhibitors. Furthermore reparixin can be utilized to interrupt the interactions between IL-8 and CXCR1/2 expressing cells (CD34+ cells, ECs and SCs). These data provide the preclinical rationale for the evaluation of combinations of drugs which can dampen the cascade of events that result when MF MKP/MKs interact with CD34+ cells and MicroE cells.
Hoffman:Summer Road: Research Funding; Merus: Research Funding; Janssen: Research Funding; Incyte: Research Funding; Formation Biologics: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.